Fugitive Emissions
09.2017 – 09.2018
This project is the result of a year-long investigation into the effects of open-pit mining in the Haut-Katanga province in the Democratic of Congo. This body of research seeks to debunk the misconceptions that metals are immobile, when in reality, ‘trace metals’ (those metals existing in concentrations less than 0.1% in the Earth’s crust) are often as prone to movement as the air and water around us. This is because trace metals are ‘chemical elements’, the building blocks of the periodic table and the Earth as we understand it. As such, the pollutants produced through open-pit mining, in the forms of aerosol emissions or waste stockpiles, cannot adequ-ately be considered the stagnant byproducts of industrial processes. In reality, these pollutants are a highly intricate composition of trace metals (such as arsenic, lead, cadmium, copper, cobalt, etc.), and because of the nature of their chemical form, are capable of persisting and migrating at local, regional, or cross-continental scales. Furthermore, because of their base form as periodic elements, they do not deteriorate into a less toxic state with their environment like other forms of pollutants, but instead perpetually cycle through earth systems once they have been extracted from the lithosphere. They drift through the air, creep through the soil, flow between rivers, are absorbed by plants and are ingested or inhaled by those populations in their proximity. They evaporate, precipitate, and at times idle for decades within the soil, only to later be remobilised by natural events such as floods or storms.
While this research is in many ways still ongoing, it has been presented in a number of forms including:
- Publication: 160-page book which includes a copy of my MA Dissertation and an archive of relevant studies which give insight into the spatial properties of trace metal movement through earth systems. The book can be found here.
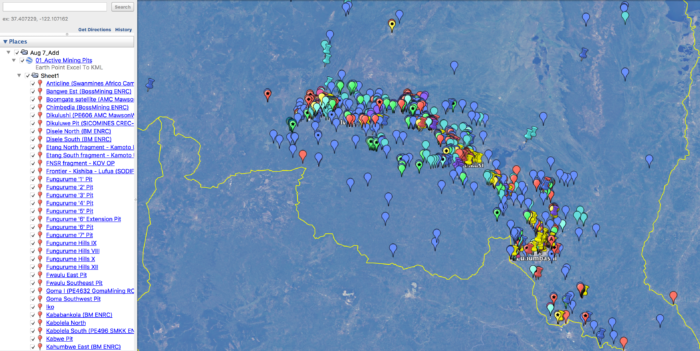
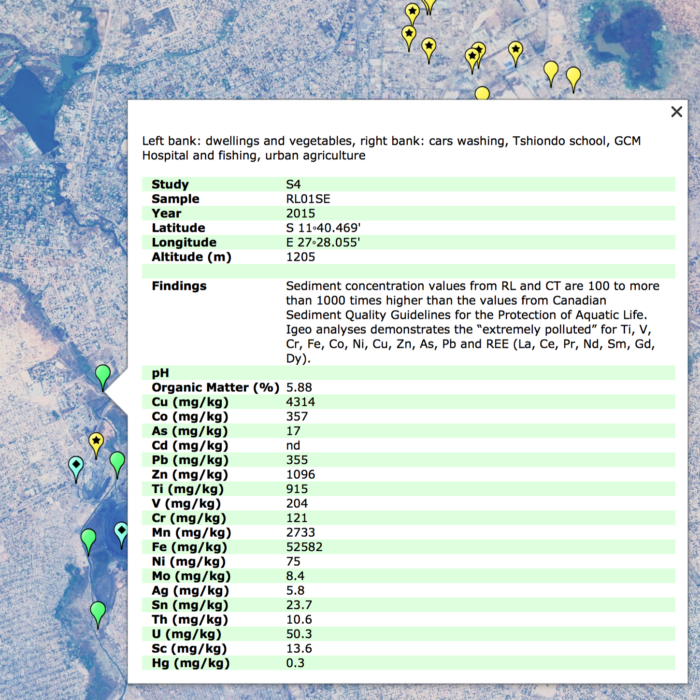
- Online GIS Archive: The location of nearly 1000 sites which compose the Democratic Republic of Congo’s Copper-Cobalt Industry. Including open-pit mining sites, smelting plants, refining plants, concentrators, processing plants, exploration drilling, artisanal mining sites, waste-rock sites, dump sites, tailing ponds, etc. These sites were plotted alongside the locations and findings of soil studies and metal concentrations. The majority of these sites are depicted in a map below.
- Exhibition: Between 27.09.18 – 30.09.18, this research was exhibited alongside other students of the Centre for Research Architecture as part of the exhibition “Para-Link.” The work was presented as four panels which included conceptual diagrams and maps which illustrated some of the central themes of the research. The exhibition also included a vibrant array of mineral samples which were sourced from different mines from the region, a looped-video showing dust escaping from an industrial plant, and a printed version of my dissertation.



The Copper-Cobalt Industry Of The Democratic Republic Of Congo
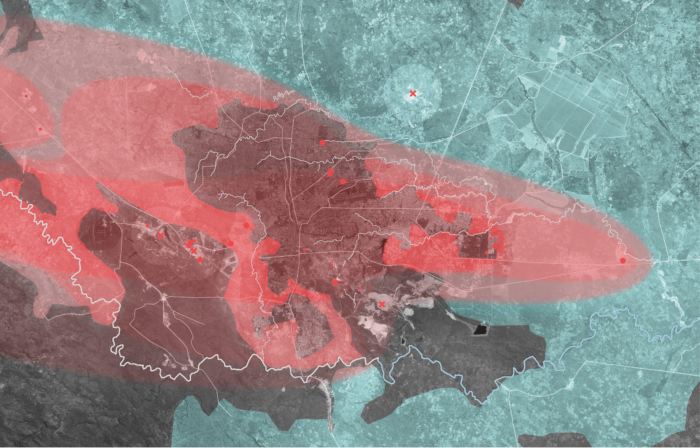
Over the course of the 2017 – 2018 period, this investigation underwent a vigorous process of analysing satellite imagery, corporate documents, and GIS databases in order to amalgamate and archive important sites within the DRC’s Copperbelt. These sites can be found in the map below, alongside a spatial representation of the pathways and concentrations of trace metal particulate emissions from smelters and other industrial mining plants. The crucial intersection between these pathways and the cultivated land of the region indicates areas particularly susceptible to be poisoned through the ingestion of produce with high trace-metal concentrations.


Trace Metals
Definition, Mobility and Toxicity
Trace Metals: “chemical elements occurring in diffuse concentrations of less than 0.1% within the earth’s crust.” [1] In trace volumes, some of these elements, such as copper and cobalt, are essential to biological growth. However, in just marginally higher concentrations they can become toxic. Other trace elements, notably arsenic, lead, and cadmium— all by-products of cobalt mining—are carcinogenic and have no fundamental role in biological growth.”
[1] T. Navratil, L. Minarik “Trace elements and contaminants” V. Cilek, R.H. Smith (Eds.), Earth System – History and Natural Variability, Vol. 8, Global Natural Cycles. EOLSS Publishers, Oxford (2011),
Trace metals naturally exist within the environment in minute concentrations, posing relatively no risk to humans and ecosystems. However, this equilibrium has been disrupted by the proliferation in recent history of regionally concentrated mining operations. Stakeholders in the mining community have made various efforts to measure and understand the impact of their operations in surrounding ecosystems. Nonetheless, due to the particularly volatile and unpredictable nature of the pollutants emitted by such mining operations, measurements used on ecosystems paint a wildly inactive and inaccurate picture of the extent of damage. Unlike other forms of pollutants which often degrade through biological and chemical processes, trace metals have longer lasting implications because they are chemical elements. As such they do not deteriorate, instead they persist for generations within the environment in perpetual motion.
Reduction
Definition, Mobility and Toxicity
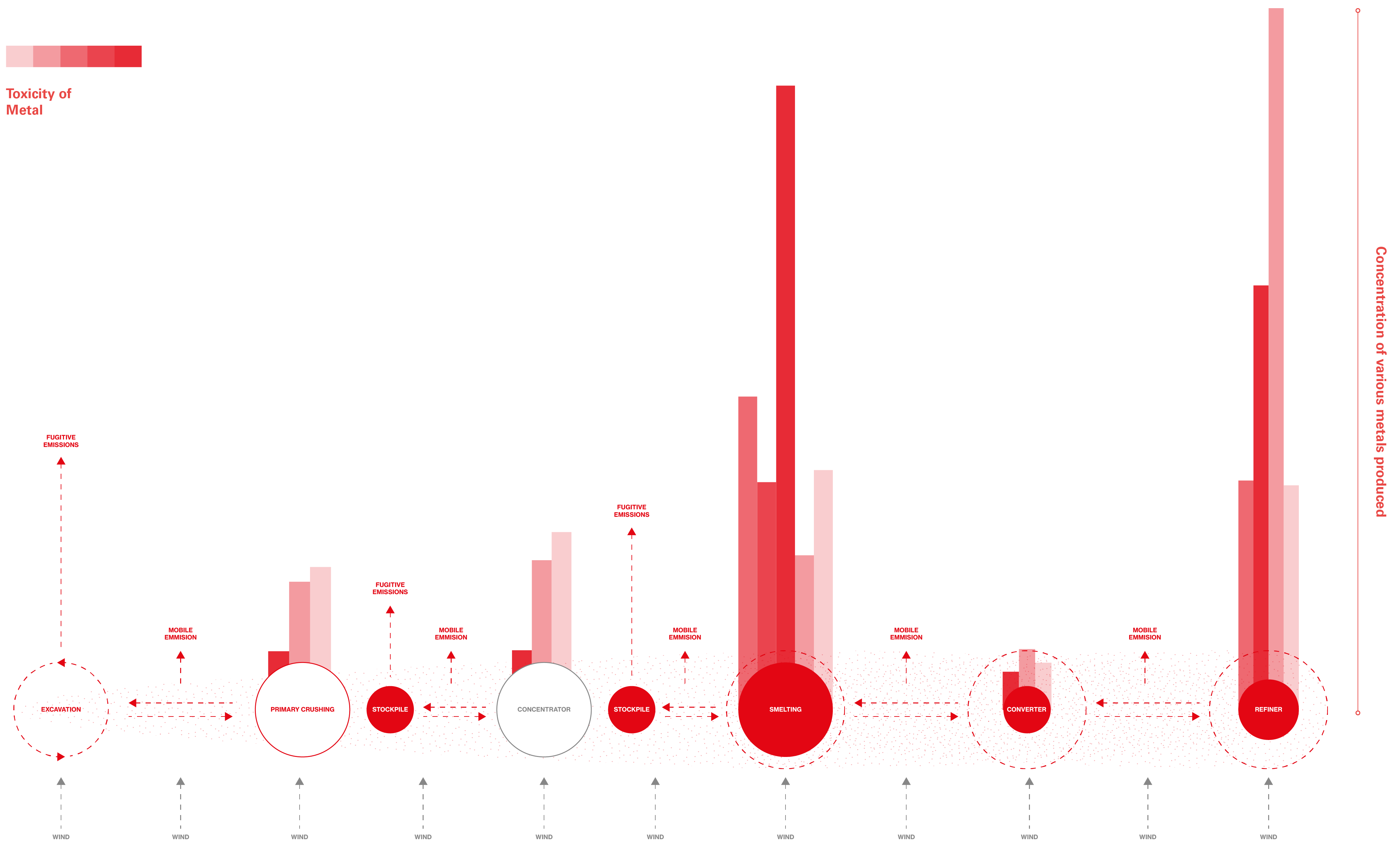
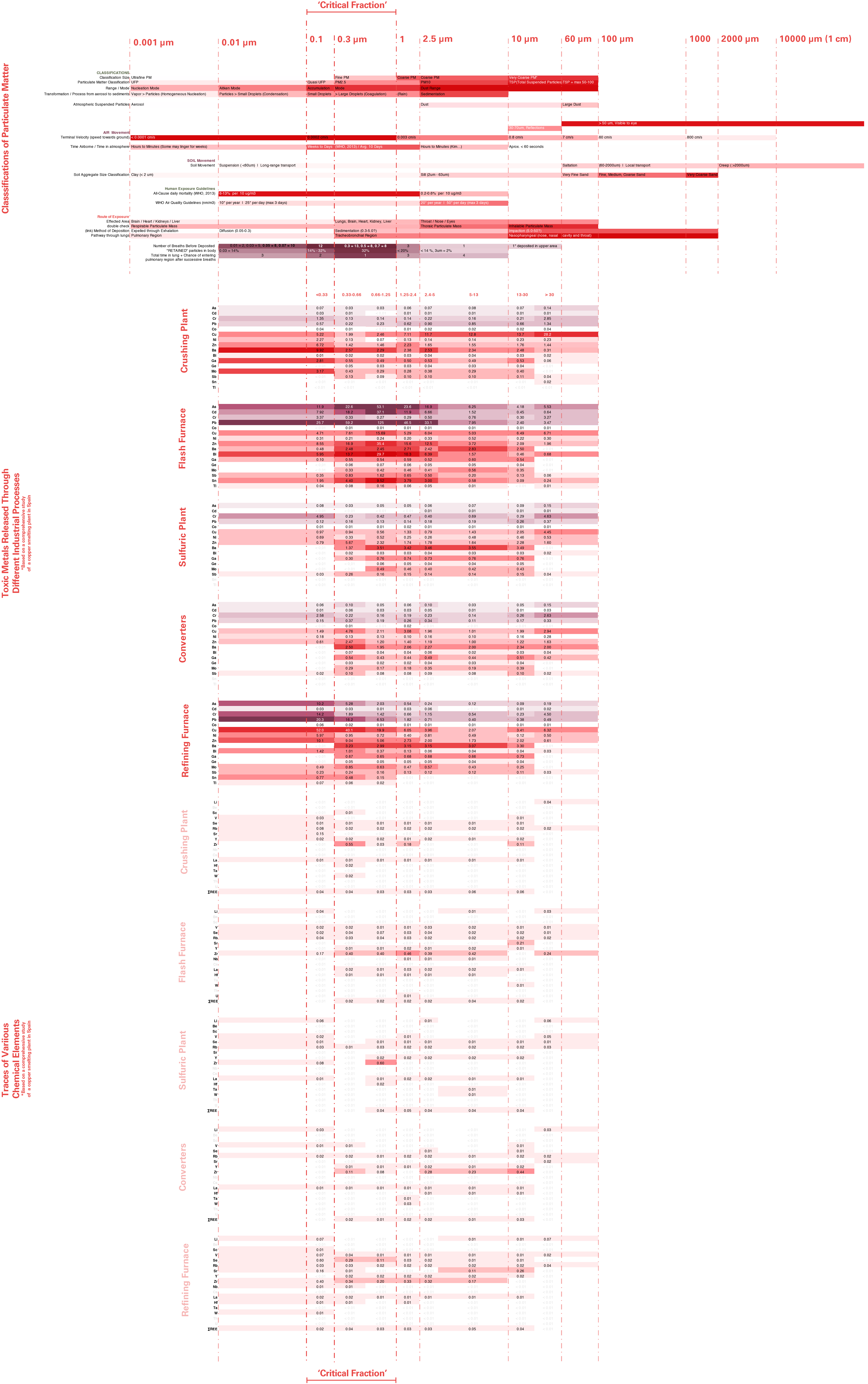
Below: A schematic diagram modelled from a comprehensive study on trace metal particulates emissions conducted in Spain. It illustrates the toxicity and volume of trace metal pollutants produced at various stages of the industrial process. It also indicates where ‘fugitive emissions’ and ‘mobile exhibition’ (produced by the movement of industrial equipment and trucks) are released. All values represented were recorded within the chimney stack after particulates had bypassed the filter.
While the extraction and processing of metals is an incredibly rigorous and technologically complex procedure, it can be understood within the context of this research as stages of reduction. Material is extracted from the lithosphere, before being pulverized, grinded, milled, leached, dissolved and melted – all in order to disentangle and separate the economically exploitable material from its superfluous geological reality. Throughout this process of reduction, mining and processing companies project an image of control over the materials they handle.
In reality, industrial-scale mining operations come with a lot of unacknowledged uncertainty. Material, which was once static for millions of years, is extracted and put through a number of highly volatile states. It is reduced to fine-grains and dust, transported large distances, subjected to wind and rainfall and heated to a gaseous-molten sludge. In the process of the materials’ reduction, input material is repeatedly transformed into product and waste. Other material, in the form of emissions, escapes these processes altogether, recirculating back into the environment they came from. When rock is reduced to a fine-granular material, it becomes easier to work with; adaptable and highly susceptible to the industrial process. It can be compacted within the back of a haul-truck, or mixed into a liquid and fed through floatation tanks. However, this reduction of a material’s base size during the industrial process also alters the relative way all forces of the external world act upon it. As dust, it can be lifted into the air, flow through rivers or seep into the soil – it is mobile – unlike its antecedent form.
Critical Fraction
[1] um = micrometer, equivalent to 0.001 millimeter
[2] Baghouse Filter: device or facility in which particulates are removed from a stream of exhaust gases (as from a blast furnace) as the stream passes through a large cloth bag – Merriam-Webster
[3] González-Castanedo et al., 2014
[4] Bakshi, Santanu & He, Zhenli & G Harris, Willie. (2014). Natural Nanoparticles: Implications for Environment and Human Health. Critical Reviews in Environmental Science and Technology. 45.
[5] González-Castanedo et al., 2014
[6] Nanoscopic: relating to ‘Nanoscale’, On a scale of 10-9 metre; having or involving dimensions of less than 100 nanometres. – Oxford Dictionary
[7] Lahoud, 2016
The largest concentration of trace metals created during the reduction of material is often a result of the smelting process. While modern technology has produced safeguards, such as filters that can collect 99.9% of trace metals, most smelters are not equipped with the latest and most expensive equipment. Significant concentrations of trace metals still escape these furnaces as fugitive emissions. For example, a study conducted in Spain sought to outline the extent of trace metal release while standard safeguards were in place. This study concluded that significant concentrations of trace metal particles (particularly those <0.33 um) [1] were capable of permeating the baghouse filter [2] in place, even though this filter met European safety standards. [3]
Why fugitive emissions bypass these safeguards is easier to grasp when one has a reasonable understanding of the size of the particles in question. Some industrial processes render particles so small that they can no longer simply be understood as ‘dust’. Dangerous metals and metalloids contained within open-pit mines are reduced to quasi-ultrafine particles (Q-UFP; <0.33 um), ultrafine particles (UFP; <0.1 um), and vapour (<0.01 um) [4] at smelters, refiners, sulphuric plants, and crushing plants. [5] These fantastically small particles are among the most dangerous fractions of particle sizes due to their likelihood of being inhaled deep into the lower respiratory tract, where they may pass directly into the bloodstream.
For companies tasked with both the movement of resources and the containment of these particulates, the scales of operation are both nanoscopic [6] and planetary. Material is constantly in motion through logistical networks, competing against the turbulent and unpredictable forces of the natural world. It is this condition of uncertainty – its migration and eventual reabsorption – that make this material so dangerous and its human rights and ecological impacts so urgent. As architect Adrian Lahoud notes, “environments loosen the bonds between cause and effect, obscuring the link between attribution, responsibility, and – potentially – justice.” [7]
Below: An amalgamation of cross-disciplinary studies regarding trace metal particulate size and their resulting classification, mobility, their likelihood of producing deleterious health effects. Of particular interest are trace metal particulates whos’ sizes are within the “critical fraction.” These particulates are cause the most harm, are capable of moving vast distances, and are the most capable of bypassing industrial filters. Furthermore, as illustrated in the seconf half of the the chart, high volumes of this size fraction are produced during several stages of the industrial process.
Movement
Through Earth
Systems
Lithosphere, Pedosphere, Atmosphere, Hydrospehere, Biosphere and Pathways of Exchange
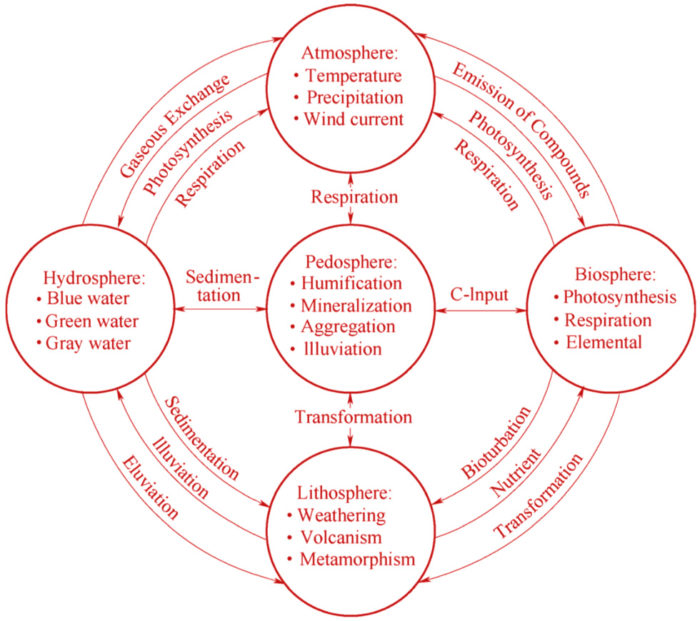
Right: The different ‘-spheres’ of earth, and possible pathways of exchange. Trace metals move between these spheres at different rates and concentrations.
Ultimately this research has set out to amalgamate a cross-disciplinary body of scientific studies and writing with regards to our present knowledge of how trace metals move through air (atmosphere), soil (pedosphere), water (hydrosphere), and biological systems (biosphere). In this research, I have outlined some of the characteristics present within our environment, which influence ‘pathways’ of trace metal pollutants. The unique pathways of individual trace metal particles are a product of a diverse set of ‘externalities’ which act on them and dictate their movement. As a product of these particles’ movement between earth’s systems, this research is inherently a cross-disciplinary and spatial exercise. These pollutants are unlike CO2 emissions – which are suspended within the atmosphere, and consequently, global in their ramifications – or oil-spills – which are localised, and biodegradable if given enough time. Comparatively, trace metals are capable of migrating regionally or globally, while also able to accumulate to toxic concentrations, producing highly-localised and detrimental effects. But perhaps most importantly, the nature of their existence as chemical elements means that they are eternal, and thus, areas affected by these pollutants are extremely difficult to remediate.

Far Left: Humidity, Monthly Rainfall, Wind Direction and Wind Speed of Likasi. Trace Metals are most mobile during the dry season between May to September each year.
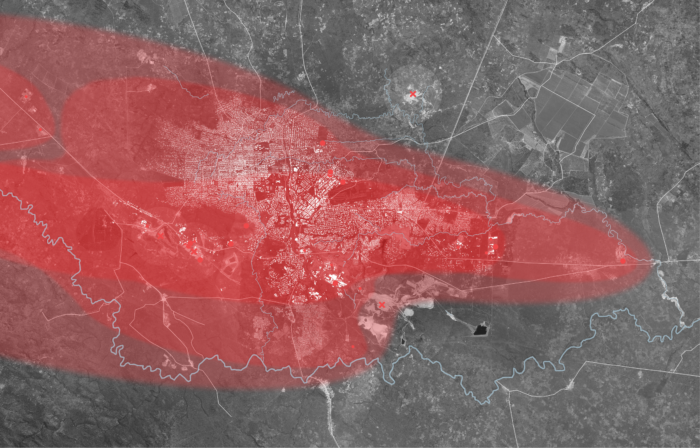
Left: Direction and Concentration of Smelting Emissions During the Dry Season. Likasi, Democratic Republic of Congo
During the dry season, trace metals originating from smelting and other industrial processes are susceptible to long-range transport. Trace metal particles can travel between continents if they are suspended in an aerosol form, however the highest concentrations of these metals are found in the soil within 10km of the point source it originated from. Significant concentrations are also found within 20km and 50km ranges within this region.
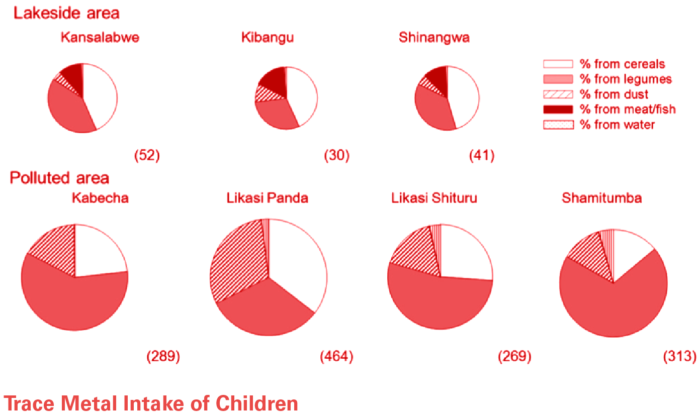
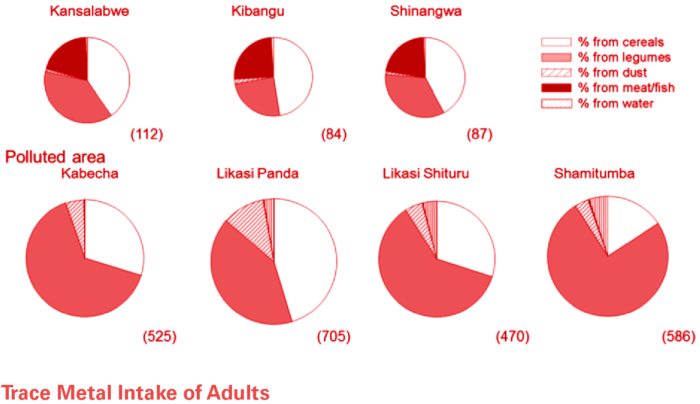
Far Left: Method (or pathway) Of Cobalt intake in Likasi vs. other communities within the DRC. Resident of Likasi (particularly children) are susceptible to cobalt intake via dust inhalation.
Left: Intersection of the Pathway of Dominant Winds and Cultivated Land. Likasi, Democratic Republic of Congo.
One of the most prominent pathways of human exposure to trace metals within the region, especially among non-occupational exposure, is through ingestion. Plants, and specifically crops, absorb vast amounts of these metals through the soil before they are past onto humans. This process of internalizing trace metals after they are ingested is known as “bio-accumulation”, and those at the top of the food chain will accumulate the largest volume of this material.
Right: Diagram depiciting the transport of trace metal particulates from a smelter to the soil. Following the release of trace metal particulates from industrial emissions, these metals enter the atmosphere and drift in the direction of predominant winds. Dependent on the humidity within the air, they can drift for days before they are eventually localised, when vapor within the air condense into rain drops which fall towards the soil. In this diagram trace metals have exited the industrial plant and passed between the atmoshpere and the hydrosphere before eventually reaching the soil. Ultimately the the distance these trace mdtal particles will travel is dependent on the relative humidity in the air. For this reason particulates can be expected to travel longer distance during the dry season.
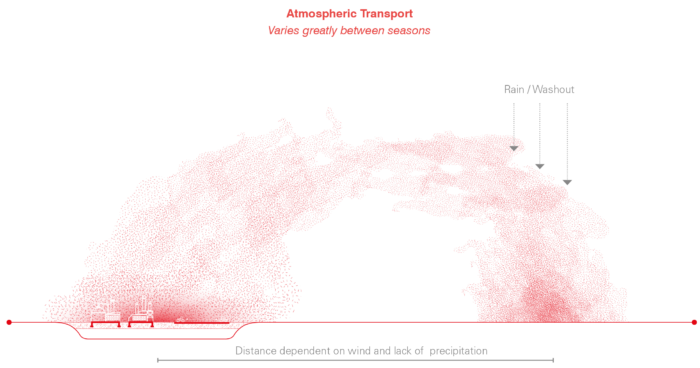
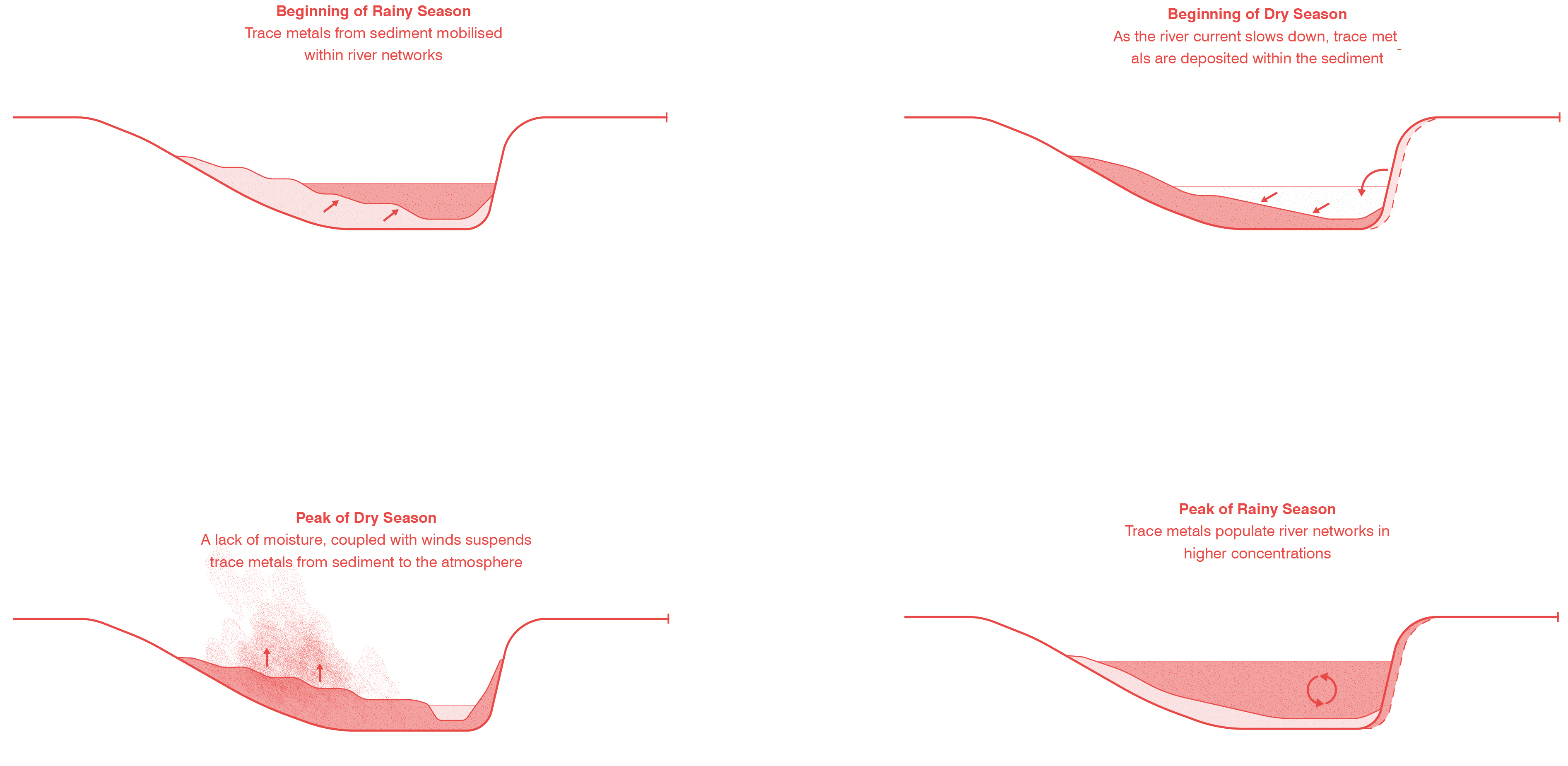
Right: Once trace metals have entered the environment, they are prone to move between earth systems in cyclical ways. Looking at the fluvial processes of river provides an example of on of the ways that tracemetals move through the hydrosphere, pedosphere and atmosphere throughout the dyanmic seasons within the region.